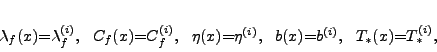Model of soil freezing and thawing
In many practical applications, heat conduction is the dominant mode of energy transfer
in a ground material. Within certain assumptions (Kudryavtsev, 1978; Andersland and Anderson, 1978)
the soil temperature ![]() can be simulated by a 1-D heat equation with
phase change (Carslaw and Jaeger, 1959):
can be simulated by a 1-D heat equation with
phase change (Carslaw and Jaeger, 1959):
The quantities ![]()
![]() and
and![]()
![]() represents the volumetric heat capacity and thermal conductivity of
soil, respectively;
represents the volumetric heat capacity and thermal conductivity of
soil, respectively; ![]() is the volumetric latent heat of fusion of water, and
is the volumetric latent heat of fusion of water, and ![]() is the volumetric water content. The heat equation (1) is
supplemented by initial temperature distribution
is the volumetric water content. The heat equation (1) is
supplemented by initial temperature distribution ![]() , and boundary
conditions at the ground surface
, and boundary
conditions at the ground surface ![]() and at the depth
and at the depth ![]() . We use the Dirichlet
boundary conditions, i.e.
. We use the Dirichlet
boundary conditions, i.e. ![]() ,
, ![]() . Here,
. Here, ![]() is the
temperature at
is the
temperature at ![]() at time
at time ![]() ;
; ![]() and
and ![]() are observed temperatures at
the ground surface and at the depth
are observed temperatures at
the ground surface and at the depth ![]() , respectively.
, respectively.
One of the commonly used measures of liquid water in the freezing soil is the volumetric
unfrozen water content (Anderson and Morgenstern, 1973; Osterkamp and Romanovsky, 1997; Watanabe and Mizoguchi, 2002; Williams, 1967). There are
many approximations to ![]() in the fully saturated soil
(Galushkin, 1997; Lunardini, 1987). The most common approximations are associated with power
or exponential functions. Based on our positive experience in Romanovsky and Osterkamp (2000), we
parameterize
in the fully saturated soil
(Galushkin, 1997; Lunardini, 1987). The most common approximations are associated with power
or exponential functions. Based on our positive experience in Romanovsky and Osterkamp (2000), we
parameterize ![]() by a power function
by a power function
![]() for
for
![]() (Lovell, 1957). The constant
(Lovell, 1957). The constant ![]() is called the freezing point
depression. In thawed soils (
is called the freezing point
depression. In thawed soils (![]() ), the amount of water in the saturated soil is
equal to the soil porosity
), the amount of water in the saturated soil is
equal to the soil porosity ![]() . Therefore, we assume that
. Therefore, we assume that
where ![]() represents the liquid pore water fraction. For example, small values of
represents the liquid pore water fraction. For example, small values of ![]() describe the liquid water content in fine-grained soils, whereas large values of
describe the liquid water content in fine-grained soils, whereas large values of ![]() are
related to coarse-grained materials in which almost all water freezes at the temperature
are
related to coarse-grained materials in which almost all water freezes at the temperature ![]() .
.
We adopt the parametrization of thermal properties proposed by (de Vries, 1963; Sass et al., 1971)
with some modifications. We express thermal conductivity ![]() of the soil and its
volumetric heat capacity
of the soil and its
volumetric heat capacity ![]() as
as
where ![]() and
and ![]() are the effective volumetric heat capacities, respectively, and
are the effective volumetric heat capacities, respectively, and ![]() and
and ![]() are the effective thermal conductivities of soil for frozen
and thawed states, respectively. For most soils, seasonal deformation of the soil
skeleton is negligible, and hence temporal variations in the total soil porosity,
are the effective thermal conductivities of soil for frozen
and thawed states, respectively. For most soils, seasonal deformation of the soil
skeleton is negligible, and hence temporal variations in the total soil porosity, ![]() ,
for each horizon are insignificant. Therefore, the thawed and frozen thermal
conductivities for the fully saturated soil are obtained from
,
for each horizon are insignificant. Therefore, the thawed and frozen thermal
conductivities for the fully saturated soil are obtained from
where subscripts ![]() ,
, ![]() , and
, and ![]() mark heat capacity
mark heat capacity ![]() , thermal conductivity
, thermal conductivity ![]() for ice at
for ice at ![]() , liquid water at
, liquid water at ![]() and solid soil particles,
respectively. Combining formulas in (4), we derive that
and solid soil particles,
respectively. Combining formulas in (4), we derive that
Hence, the thermal properties ![]() and
and ![]() are expressed by only four variables such
as
are expressed by only four variables such
as ![]() ,
, ![]() ,
, ![]() , and
, and ![]() .
.
Evaporation from the ground surface and from within the upper organic layer can cause
partial saturation of upper soil horizons (Kane et al., 2001; Hinzman et al., 1991). Therefore, formulae
(4) need not hold in the presence of live vegetation and within organic soil
layers, and possibly not within organically enriched mineral soil (Romanovsky and Osterkamp, 1997).
Besides organic and organically enriched mineral soil layers, there are other horizons,
all of which can have distinctive physical properties, texture and mineral composition.
We assume that there are several horizons, namely: an organic layer, an organically
enriched mineral soil layer, and a series of mineral soil layers. We assume that physical
and thermal properties do not vary within each horizon, and hence ![]() ,
, ![]() ,
,
![]() can be assumed to be constants within each soil horizon:
can be assumed to be constants within each soil horizon:
where the index ![]() marks the index of the soil layer. Table 1 shows a typical
soil horizon geometry and the commonly occurring ranges for the porosity
marks the index of the soil layer. Table 1 shows a typical
soil horizon geometry and the commonly occurring ranges for the porosity ![]() , thermal
conductivity
, thermal
conductivity ![]() and the coefficients
and the coefficients ![]() ,
, ![]() parameterizing the unfrozen
water content.
parameterizing the unfrozen
water content.